
When a generic drug gets tentative approval from the FDA, it doesn’t mean it’s ready to hit pharmacy shelves. It means the agency has confirmed the drug is safe, effective, and made to the right quality standards-but something is still blocking its release. And that something often takes years to clear. For patients waiting for affordable alternatives to expensive brand-name drugs, these delays aren’t just bureaucratic footnotes-they’re real barriers to care.
What Tentative Approval Actually Means
Tentative approval isn’t a halfway mark. It’s a full scientific green light. The FDA has reviewed the chemistry, manufacturing, and clinical data for the generic version and found it identical in performance to the brand-name drug. The problem? The original drug still has patents or exclusivity rights protecting it from competition. Until those expire, the FDA can’t give final approval-even if the generic is ready to go. This system was created by the Hatch-Waxman Act of 1984 to balance innovation and access. It lets generic manufacturers prepare in advance, so once the patent expires, they can launch immediately. But in practice, that immediate launch rarely happens. The gap between tentative approval and market entry can stretch for years.Review Cycles: The Long Road Through the FDA
One of the biggest reasons for delays is how many times the FDA sends the application back for fixes. Before 2012, most generic applications went through nearly four rounds of review before approval. Even after the Generic Drug User Fee Amendments (GDUFA) were introduced to speed things up, the average was still 3.2 cycles in 2022. The most common reasons for rejection? Incomplete or weak chemistry, manufacturing, and controls (CMC) data. That’s about 35% of all issues. Manufacturers often don’t provide enough detail on how the drug is made-things like particle size, dissolution rates, or stability under different temperatures. Without this, the FDA can’t be sure the generic will behave the same way in the body as the brand. Bioequivalence studies are another major hurdle. About 28% of deficiencies come from poorly designed or reported studies. These tests prove the generic drug releases the same amount of medicine into the bloodstream at the same rate as the brand. If the study doesn’t meet FDA standards, the application gets kicked back. And because these studies can take months to redo, the whole timeline slips.Factory Problems: The Hidden Bottleneck
Even if the science is perfect, the manufacturing site can derail everything. In 2022, 41% of complete response letters from the FDA were due to facility issues. That’s not just about cleanliness-it’s about systems. The most frequent inspection findings? Inadequate quality control systems (63% of facility-related issues), failure to properly monitor environmental conditions like humidity and temperature (29%), and unqualified or poorly maintained equipment (24%). These aren’t minor oversights. If a factory can’t prove it consistently produces the same quality batch after batch, the FDA won’t approve it. And getting a facility re-inspected after a failure can take six months or more. Many smaller generic makers don’t have the resources to fix these problems quickly, which pushes their launch dates further out.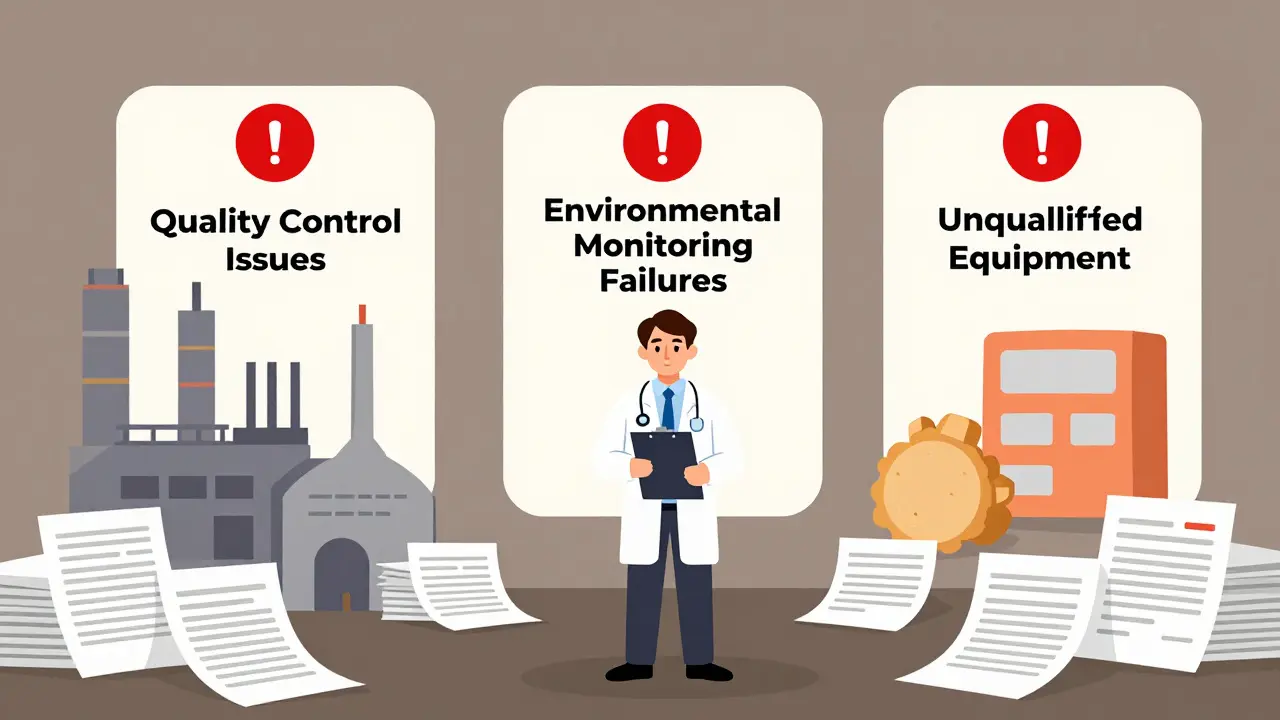
Patent Litigation: The Legal Wall
This is where things get messy. When a generic company files an ANDA and says a brand-name patent is invalid or won’t be infringed (called a Paragraph IV certification), the brand manufacturer can sue. And when they do, the FDA is legally required to delay final approval for up to 30 months. That’s not a suggestion. It’s a hard stop. Even if the generic has tentative approval, even if the FDA says it’s good to go, the clock stops until the court case is resolved. Between 2010 and 2016, 68% of tentatively approved generics were held up by these lawsuits. And it’s not just about real patent disputes. Brand companies often file lawsuits even when the patent is weak or about to expire anyway-just to buy time. This tactic is so common, it has a name: “evergreening.” Then there’s the “citizen petition” trick. Brand companies submit formal requests to the FDA asking them to delay approval on technical grounds-like claiming the bioequivalence method is flawed. Between 2013 and 2015, the FDA received 67 of these petitions. Only three were approved. But they still worked: each one added months to the timeline.Complex Drugs: The Tougher Targets
Not all generics are created equal. Simple pills? Easy. Inhalers, creams, injectables, or extended-release tablets? Not so much. Complex generics require more testing, more manufacturing precision, and more FDA scrutiny. In 2022, complex products like topical creams or inhalers went through 2.3 times more review cycles than regular oral pills. The FDA’s own data shows these products take 14 months longer on average to get approved. And even after approval, manufacturers often delay launching them. Why? Because scaling up production for complex formulations is expensive and risky. One bad batch can cost hundreds of thousands of dollars. Many companies wait until they’re sure they can make enough to meet demand before they launch.
Market Economics: Why Some Generics Never Launch
Here’s the uncomfortable truth: sometimes, a generic drug gets tentative approval-and then just sits there. No launch. No sale. No patient benefit. About 30% of tentatively approved generics never make it to market. For drugs with annual U.S. sales under $50 million, that number jumps to 47%. Why? Because the profit isn’t worth the risk. If only one or two companies make a generic, prices stay high. A 2019 JAMA study found that even after generic entry, prices stayed above 80% of the brand price for two full years if there was only one competitor. That means no incentive for other companies to enter the market. No competition = no price drop = no reason to launch. Manufacturers also wait for the “right time.” Maybe they’re waiting for a competitor to launch first, or for the brand to drop its price. Maybe they’re waiting for a better deal with distributors. The FDA doesn’t control these decisions-but they’re a huge part of why tentative approval doesn’t lead to affordable drugs.What’s Being Done to Fix It
The FDA knows the system is broken. That’s why they launched the Competitive Generic Therapy (CGT) pathway in 2017. Drugs with little or no generic competition get priority review. About 78% of CGT-designated drugs received tentative approval within eight months-much faster than the usual 18. GDUFA III, which runs from 2023 to 2027, has even bigger goals: raise first-cycle approval rates from 28% to 70% by 2027, and cut review times for priority drugs to eight months. They’re also targeting the top 102 high-priority tentative approvals with faster reviews. So far, 67% of those got final approval within a year, compared to just 34% for others. Legislation like the CREATES Act is trying to stop brand companies from blocking access to samples needed for testing. The Affordable Drug Manufacturing Act aims to bring more production back to the U.S. to reduce supply chain risks. But progress is slow. The FDA’s own 2023 report admits that patent litigation, complex products, and resource limits will keep delays going through at least 2025.What This Means for Patients
If you’re waiting for a cheaper version of your medication, understanding these delays helps explain why it’s taking so long. It’s not that the generic doesn’t exist. It’s that the system is clogged with legal, manufacturing, and economic roadblocks. The good news? More generics are getting approved than ever before. In 2022, the FDA approved nearly 1,000. The bad news? The median time from tentative approval to market launch is still 16.5 months. That’s over a year and a half of waiting-even after the science is done. Until the patent system is reformed, manufacturing capacity expands, and economic incentives align, patients will keep paying more than they should. Tentative approval isn’t the finish line. It’s just the beginning of a long, complicated race.What is the difference between tentative approval and final approval for generics?
Tentative approval means the FDA has confirmed the generic drug meets all scientific and quality standards for safety, effectiveness, and manufacturing. But final approval can’t be granted until any patents or exclusivity rights on the brand-name drug expire. Final approval allows the generic to be legally sold in the U.S. market.
Why do some generics with tentative approval never reach the market?
About 30% of tentatively approved generics never launch. This often happens when the drug targets a small market with low sales (under $50 million annually), making it unprofitable for manufacturers to invest in production and distribution. Other reasons include manufacturing challenges, especially with complex drugs, or strategic delays by companies waiting for better market conditions.
How long does it typically take from tentative approval to market launch?
As of 2022, the median time from tentative approval to market launch was 16.5 months. For complex drugs like inhalers or creams, delays can stretch beyond 12 months after patent expiration. Patent litigation can add even more time-sometimes years-before a generic can be sold.
Can the FDA approve a generic drug before the brand’s patent expires?
No. Even if a generic has tentative approval, the FDA cannot grant final approval until all patents and exclusivity periods on the brand-name drug have expired. This is a legal requirement under the Hatch-Waxman Act. The only exception is if a court rules the patent is invalid or not infringed, which can allow early approval.
What role do citizen petitions play in delaying generic drugs?
Brand-name companies often file citizen petitions asking the FDA to delay generic approval by challenging the scientific basis for bioequivalence or other requirements. Between 2013 and 2015, 67 such petitions were filed, and while only three were granted, each one added an average of 7.2 months to the approval timeline. These petitions are often used strategically to extend market exclusivity without winning in court.
Are complex generics harder to get approved than simple pills?
Yes. Complex generics-like inhalers, topical creams, injectables, or extended-release tablets-require more detailed data, stricter manufacturing controls, and more review cycles. On average, they go through 2.3 times more review cycles than simple oral pills and take 14 months longer to get approved. The FDA has issued special guidance for these products, but implementation remains challenging.



Wesley Pereira
January 7, 2026 AT 00:36So let me get this straight-FDA says ‘yo, this generic is solid,’ but we’re stuck waiting because some pharma giant holds a patent on a molecule they didn’t even invent? Classic evergreening. We’re paying $500 for a drug that costs $2 to make, all because lawyers got more power than scientists. 🤡
Susan Arlene
January 7, 2026 AT 06:09the whole system is just broken tbh. we got the medicine ready but no one lets us sell it. it’s like baking a cake and being told you can’t serve it until the original baker says it’s ok. who even made this rule?
Jeane Hendrix
January 9, 2026 AT 00:24CMC deficiencies are the silent killer of generic pipelines. I’ve reviewed dozens of ANDAs where the dissolution profile was either missing or statistically underpowered. And don’t even get me started on the particle size distribution data-half the time it’s just a screenshot from a microscope with no calibration. The FDA’s not being nitpicky; they’re trying to prevent another Valsartan contamination scenario. But yeah, the process is glacial.
Saylor Frye
January 9, 2026 AT 06:17Interesting. So we’re talking about a system designed to incentivize innovation that now just incentivizes legal gamesmanship. Who wrote this again? Oh right-the same people who think ‘patent evergreening’ is a feature, not a bug.
Tiffany Adjei - Opong
January 9, 2026 AT 15:32Wait, so you’re saying the FDA approves a drug but then lets Big Pharma block it with lawsuits? That’s not regulation-that’s corporate capture. And the citizen petitions? Pure extortion. They file them knowing they’ll be denied, but they still delay launch for 7+ months. That’s not a loophole-it’s a weapon.
Molly McLane
January 10, 2026 AT 15:03For anyone wondering why your insulin or asthma inhaler is still expensive-this is why. Complex generics aren’t just harder to make, they’re harder to finance. Small manufacturers don’t have the cash to sit through 3 FDA rejections and a 2-year patent fight. We need public funding for these, not just ‘market incentives.’
Lily Lilyy
January 11, 2026 AT 11:21I just want to say thank you to everyone who works so hard to make affordable medicine possible. Even when the system is broken, you’re still showing up. I believe in you. 💪❤️
Ryan Barr
January 12, 2026 AT 06:58Patent litigation delays = $20 billion in lost savings annually. End of story.
Mukesh Pareek
January 12, 2026 AT 21:08India produces 40% of the world’s generics. Why does FDA still treat Indian plants like they’re crime scenes? The same factories that supply WHO and EU get shut down for ‘humidity issues.’ Double standard. The US wants cheap drugs but won’t let the people who make them succeed.
Cam Jane
January 13, 2026 AT 23:55Let me break this down simply: if a generic is tentatively approved, it means the science is done. The rest? Politics, profit, and paperwork. The FDA can’t force a company to launch-even if they have the drug in hand. And that’s why you see 30% of approved generics just… vanish. No one wants to be the first to drop price in a tiny market. So no one drops price. And patients pay. It’s a tragedy wrapped in a regulatory loop.
Joann Absi
January 15, 2026 AT 00:31THE SYSTEM IS RIGGED 😭💸 PATENTS AREN’T PROTECTION THEY’RE EXTORTION 🚨 BRAND PHARMA ISN’T INNOVATING-THEY’RE JUST SITTING ON A PATENT LIKE A DRAGON ON A GOLD PILE 🐉 And now they’re using CITIZEN PETITIONS like a spam bot to delay generics? I’m so mad I could cry. We need a revolution. Or at least a very angry tweet storm. 🤬🇺🇸
Matt Beck
January 16, 2026 AT 19:16It’s not about science anymore. It’s about who owns the right to say ‘yes’ or ‘no’ to life-saving medicine. The FDA is just the referee in a game where the players wrote the rules. And we’re all just watching while our prescriptions get more expensive.
Gabrielle Panchev
January 16, 2026 AT 22:07And yet, despite all this-despite the 3.2 review cycles, the 41% facility rejections, the 68% litigation delays, the 30% non-launch rate, the $20 billion in lost savings, the evergreening, the citizen petitions, the manufacturing bottlenecks, the complex drug barriers, the lack of incentives for low-volume drugs, the fact that the FDA’s own 2023 report admits the system won’t meaningfully improve until 2025-somehow, we still believe that the free market will fix this. We’re not just naive-we’re willfully delusional.